- Nick Water DR
- Published
- Updated December 3, 2025
- Articles, Guides & Maintenance, Water contaminants, Water Filtration
Activated carbon water filters – a false sense of security ?
Have you just bought a carbon filter ?
Activated carbon has always been a popular method for treating water because it’s relatively cheap and constructive to taste and smell which is great superficially but as we are increasingly aware this can serve as a trojan horse masking our senses to assume the water is clean while not removing some more sinister contaminants …
Some of the traditional jug AC filters have been facing scrutiny lately for misleading marketing about what contaminants they remove …. Aggressive marketing of benefits by recent well known undersink celebrity brands , particularly now they are paying to feature in the search criteria for other methods of filtration like reverse osmosis are muddying the unregulated waters of perceived performance and reality !
Do they work ?
Carbon filters are everywhere in jugs, tap mounted , under-sink systems, fridges and even shower heads . They’re affordable, convenient and improve taste and smell. Activated carbon filters work by adsorption not filtration . It is made from materials rich in carbon (coconut shells, coal, wood ) that are “activated” by heating them with steam or chemicals at very high temperatures (800–1000 °C) . This process creates millions of tiny pores (like a sponge on steroids).
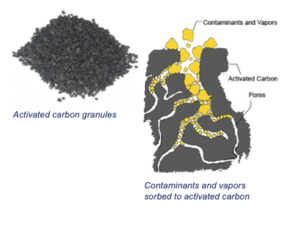
Huge internal surface area (~800–1500 m² per gram)
Micro-, meso-, and macropores
Surface chemistry that binds organic molecules
These carbon filters fall into 2 main categories granular activated carbon (GAC) loose granules in a cartridge or carbon block which in general work better where the granules are compressed.
Great for removing “some” contaminants …
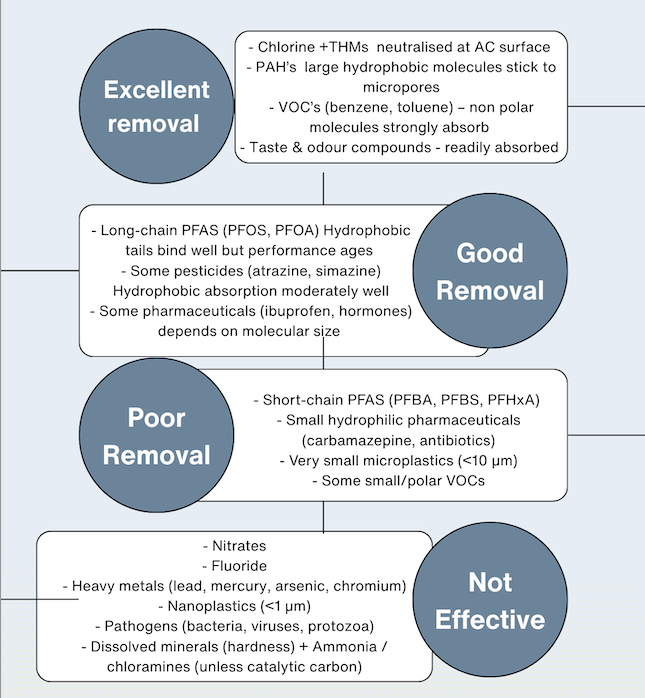
Activated carbon is genuinely effective at removing several unwanted and potentially harmful substances — most notably chlorine, which is added as a disinfectant throughout the UK’s water network. Many people recognise it immediately by its strong smell or taste, especially when water companies increase doses during system maintenance or flushing events. Unlike many other drinking-water contaminants, residual chlorine has no legally enforced numeric upper limit in UK law; instead, water suppliers follow best-practice guidance, generally keeping levels around 0.5 mg/L or lower, which regulators deem acceptable for public health. Activated carbon excels here: it strips chlorine efficiently, improving taste and eliminating the harsh smell .
While chlorine is an essential disinfectant, higher doses or longer contact times can increase the formation of disinfection by-products (DBPs) — mainly trihalomethanes (THMs) and haloacetic acids (HAAs). These form when chlorine reacts with natural organic matter (NOM) such as decaying vegetation, biofilms inside old pipes, or organic residues in reservoirs and treatment works and even in household plumbing if water remains stagnant.
THMs and HAAs are considered carcinogenic with long-term exposure, which is why they are tightly regulated in the UK. Activated carbon helps reduce this risk by removing both chlorine and some fraction of the organic precursors, preventing the formation of new DBPs at the carbon surface.
AC also provides a line of defence against carcinogenic substances PAHs and VOCs ( benzene + toluene ) adsorbing these hydrophobic molecules that bind tightly to the carbon’s microporous surface .
Works OK on a few more …
Long-chain PFAS such as PFOS and PFOA have strongly hydrophobic “tails” that bind relatively well to carbon, so they are among the PFAS types most effectively reduced but performance declines as the carbon becomes saturated, and short-chain PFAS generally slip through more easily.
Some pesticides, including atrazine and simazine, are also moderately hydrophobic, allowing activated carbon to capture them through van der Waals forces within micro- and mesopores; however, efficiency varies with water chemistry and carbon grade.
Certain pharmaceutical residues — such as ibuprofen or hormone-like compounds — can also be adsorbed depending on their molecular size and polarity: small, moderately hydrophobic molecules bind better, while very polar or very small pharmaceuticals tend to pass through
many pass straight through ….
AC struggles with contaminants that are small, highly water-soluble, or electrically charged. This means AC provides little to no removal of nitrates, fluoride, arsenic, lead, mercury, chromium, and other dissolved metals, which simply pass through because they don’t adsorb onto carbon. It also performs poorly on short-chain PFAS (such as PFBA, PFBS, PFHxA), which are too small and too hydrophilic to stick to carbon, as well as many small or polar pharmaceuticals including carbamazepine and certain antibiotics. Activated carbon also cannot mechanically filter out very small microplastics (<10 µm) or nanoplastics (<1 µm) because its pore structure is not a physical barrier like a membrane. It offers no protection against pathogens, meaning bacteria, viruses, and protozoa are not removed or inactivated—and in fact, microbes can grow on the carbon surface over time. AC also cannot remove dissolved minerals, hardness, ammonia, or chloramines unless a special catalytic carbon is used
Performance is not static
Many manufacturers publish impressive-looking contaminant-removal figures (e.g., “95% chlorine reduction,” “1-micron filtration”), but these results come from ideal laboratory conditions: brand-new cartridges, clean test water, slow controlled flow rates, and stable pressure. Real household conditions rarely match this. Mains pressure fluctuates, flow is intermittent, water chemistry varies by region, and users often delay replacing cartridges — all of which reduce real-world performance.In engineering practice, the key performance factor is Empty Bed Contact Time (EBCT) — how long water actually remains in contact with the carbon media. Longer contact time and larger carbon volume yield better adsorption. A filter’s “maximum pressure” rating only indicates that the housing can withstand that pressure; it does guarantee effective contaminant removal at high flow. Most compact under-sink units simply lack the bed depth and contact time required for harder-to-remove contaminants, and as cartridges age, they also develop preferential flow paths (water bypassing parts of the media).
Using your common sense – drinking water utilisation is statistically less than 10 % of overall usage for a kitchen sink … remembering how important contact time helps performance undersink solutions may suffer from quickly filling the pores with contamination . Jugs are frustrating due to the smaller capacity volumes but intuitively will provide better quality absorption over time
Maintenance matters because adsorption capacity is finite: pores gradually fill, performance declines, and eventually breakthrough occurs. At that point the filter may remove far less than claimed, or even release previously adsorbed compounds. Compounding this, moist carbon that has stripped chlorine from the water becomes an ideal habitat for microbes — biofilms form, bacteria multiply, and can be released downstream, sometimes making filtered water microbiologically worse than the tap water entering the system.
In short: published test results represent best-case scenarios, not guaranteed household performance. There is no legal requirement for replacement intervals to be tied to actual contaminant breakthrough. Without timely maintenance and realistic expectations, a carbon filter can stop improving water quality — and may even start degrading it.

Part of the solution NOT the solution !
Activated carbon filters unquestionably play an important role in improving household drinking water: they are inexpensive, widely available, and highly effective at removing chlorine, many tastes and odours, and a subset of organic chemicals. But they are only one stage in a truly secure, long-term water-quality strategy not a silver bullet. Their performance declines with use, their adsorption capacity is finite, and they provide no reliable protection against many priority contaminants . Understanding these limitations helps prevent a false sense of security.
Although the cartridge itself is cheap, effective protection requires proactive maintenance: replacing filters on schedule (or sooner), monitoring flow decline, and recognising that lab-verified “removal percentages” reflect ideal test conditions rather than real household plumbing. Over-extension of a cartridge means poorer performance, possible contaminant breakthrough, and increased bacterial growth inside the media all of which compromise water quality rather than improve it.
In essence: activated carbon is an excellent first barrier, but only when it is large enough, slow enough, and maintained well enough to work as designed. With clearer understanding of what carbon filters can — and cannot — do, households can make informed choices, combine technologies intelligently, and build multi-layered protection against chemical and microbial risks in the water they drink, cook with, bathe in, and breathe every day.
Understanding the above and then implementing a strategy to include these barriers at key places across your home are an important part of our consultation analysis and we encourage you to book man appointment to better understand your individual requirements..


About the Author
Nick Smith | Founder | The Water Dr. & Cellthyhomes
Nick has dedicated years to studying building biology, healthy living environments, and the impact of environmental toxins on inflammation.
Whilst regulations for UK drinking water are slow to adapt, & influenced by conflicts of interest, Nick conduct comprehensive research on global regulations & scientific literature to offer water filtration solutions that provide clean drinking water free from all harmful contaminants.