
- Nick Water DR
- Published
- Updated November 20, 2025
- Guides & Maintenance, Reverse Osmosis
Hungry water and the universal solvent
A water molecule (H₂O) looks a little like Mickey Mouse’s head
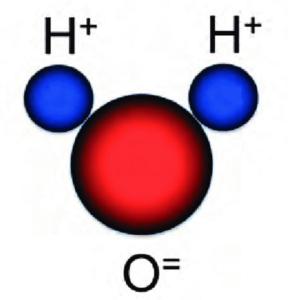
Water’s superpowers come down to its shape and a bit of electrical wizardry. Picture a water molecule (H₂O) as a tiny V: oxygen in the middle, two hydrogens sticking out like arms. Oxygen is greedy – it hogs electrons more than hydrogen, giving the oxygen end a slight negative charge and the hydrogens a slight positive one. This makes water polar, like a mini-magnet.
That polarity is key. Most things we dissolve – salts, sugars, minerals – break into charged particles called ions. Positive sodium ions (Na⁺) from table salt get swarmed by water’s negative oxygen sides, while negative chloride ions (Cl⁻) bond to the positive hydrogens. Boom: the salt vanishes into solution, carried along like passengers on a bus. Water doesn’t just dissolve ionic stuff; its ability to form hydrogen bonds (weak attractions between molecules) lets it tackle sugars, gases, even some oils. No other liquid matches this versatility – hence “universal solvent.”
Why so much absorption? Water’s bonds are just right: strong enough to pull things apart, flexible enough not to let go. In a glass, this means your squash mixes instantly. On a bigger scale, it’s why rivers carve valleys and why our drinking water arrives laced with nature’s cocktail – and sometimes its cocktails of trouble.
Water’s Natural Journey: From Cloud to Cup, Picking Up Friends and Foes
Rain starts life pure, distilled by the sky. But as it falls through CO₂-rich air, it forms weak carbonic acid, giving it a slight tang (pH around 5.6). Hitting the ground, this mildly acidic water begins its solvent spree, seeping through soil and rock. In the UK’s varied landscape – from granite moors in Dartmoor to limestone dales in Yorkshire – it dissolves what it meets: calcium and magnesium for that “hard” water feel in the southeast, or peaty tannins for the amber hue of Scottish burns.
This is mostly good: minerals like calcium make water taste rounded and help buffer acidity. But water’s eagerness means it also hauls contaminants. In the UK, where 70% of drinking water comes from surface sources like rivers and reservoirs, this journey often picks up human handiwork.
Take nitrates, a classic farmyard hitchhiker. Fertiliser runoff from fields in the East of England – think Norfolk’s arable heartlands – leaches into aquifers and rivers. Water’s polarity loves nitrate ions (NO₃⁻), so it carries them straight to treatment works. In 2023, over 20 UK water companies breached nitrate limits in raw supplies, forcing extra blending or ion-exchange tech to keep tap levels under 50 mg/L (the legal max) it shows how water’s solvent nature amplifies agriculture’s footprint.

Then there’s lead, a legacy villain. In Victorian-era cities like Manchester or Glasgow, old pipes leach Pb²⁺ ions into water as it flows. Lead’s positive charge makes it easy pickings for water’s negative ends. A 2021 study found high lead in 14 English schools’ taps, some over 100 µg/L – way above the 10 µg/L guideline – because stagnant water in lead-lined pipes dissolved more over time. Today, UK water firms flush lines and add orthophosphate to coat pipes, but it underscores water’s relentless pull on metals.
Don’t forget cryptosporidium, a microscopic parasite that hit headlines in 2024’s Devon outbreak. This bug rides livestock manure into rivers like the River Dart, surviving chlorination because water carries it intact until UV treatment zaps it. Over 100 cases sickened locals, traced to raw water contamination in a catchment grazed by cattle. Water’s ability to suspend particles and microbes without breaking them down is a double-edged sword.
And the hot topic of 2025: PFAS, or “forever chemicals.” These synthetic fluorocarbons from non-stick pans, firefighting foams, and industry don’t dissolve easily elsewhere, but water’s hydrogen bonds snag their polar ends. In January 2025, The Guardian revealed hotspots in England’s raw supplies – 199 samples over 100 ng/L for individual PFAS, especially near RAF bases and chemical plants in the Midlands and southeast. The Drinking Water Inspectorate (DWI) ordered action at sites serving 6 million, including granular activated carbon filters to strip them out. PFAS exemplify how water absorbs persistent pollutants, accumulating them like an unwilling courier.
The Purity Paradox: When Clean Water Gets “Hungry”
We love pure water – or so we think. Enter reverse osmosis (RO), the gold standard for home filtration. RO forces water through a semi-permeable membrane tighter than a footballer’s kit, rejecting salts, microbes, and chemicals. Output? Ultra-pure H₂O at 1–10 mg/L TDS – a far cry from nature’s 100–500 mg/L norm.
But here’s the twist: this purity makes water aggressive. In chemistry, solutions seek equilibrium. Natural water is saturated, like a sponge full to bursting. RO water? It’s a dry sponge in a rainstorm – undersaturated, with huge capacity left to dissolve. Without buffering bicarbonates (stripped by the membrane), it absorbs CO₂ from air, dropping pH to 5–6 and tasting sharp. Worse, it leaches from everything: copper from pipes, plastic from bottles, even silica from glass.
In the UK, where hard water dominates the south and east, RO owners notice kettles fur-free at first… then pitting from the “hungry” attack on metal. It’s called “demineralised aggression” – the water’s polarity, unchecked, turns inward. No wonder it feels flat: our palates evolved for mineral-laden water, not lab-grade distillate.
What Generic RO Systems Use to Fix the Problem
After reverse osmosis gives you ultra-pure, oddly flat water, the final stage adds minerals back to make it taste right again.
Basic systems use pure calcite (crushed limestone) → hard-water taste, 100–230 mg/L TDS and noticeable kettle scale.
A step up is calcite + magnesium blends → richer and silkier, 150–300 mg/L, but still some scaling.
Very cheap units fit ceramic balls → only 10–60 mg/L added, light and clean but usually still feels thin.
“Alkaline” cartridges go big → 250–600 mg/L and pH 9–10+, bold (often soapy) flavour and rapid limescale.
Balanced modern cartridge → perfect 60–120 mg/L TDS at neutral pH, crisp premium-spring taste, almost no scale and always consistent.
A few systems simply blend 10–20 % of your original tap water back in → familiar local taste, but you also keep the original hardness and any regional quirks.

A Thirsty World: Lessons from the UK Tap
Water’s universal charm is its curse too. From polar pull to natural leaching, it’s Earth’s ultimate mixer
Water is the quiet architect of life itself: transparent, tasteless, seemingly simple—yet it possesses properties that border on the magical. It is the only natural substance that exists in all three physical states—liquid, solid, and gas—within the narrow temperature range that sustains life on Earth. Its extraordinary ability to dissolve more substances than any other liquid makes it the universal solvent, the medium in which the intricate chemistry of biology unfolds.
We are indeed mostly water , roughly two-thirds of the human body is H₂O. Our blood is mostly water, carrying oxygen and nutrients to every cell. Our brains float in it, cushioned and cooled. Our muscles depend on it for strength, our joints for smooth motion, our skin for suppleness. Even our bones, which seem so solid, are about 31% water. We are, in the most literal sense, animated oceans—walking, thinking, feeling bodies of water shaped into human form.


About the Author
Nick Smith | Founder | The Water Dr. & Cellthyhomes
Nick has dedicated years to studying building biology, healthy living environments, and the impact of environmental toxins on inflammation.
Whilst regulations for UK drinking water are slow to adapt, & influenced by conflicts of interest, Nick conduct comprehensive research on global regulations & scientific literature to offer water filtration solutions that provide clean drinking water free from all harmful contaminants.




